Perspective
Beyond the Label: A Patient-Centred Approach to Polycystic Ovary Syndrome
Lara Briden1
ABSTRACT
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder affecting women of reproductive age. Importantly, it is not one disease with a single pathophysiology but is instead a heterogeneous syndrome with several underlying biological mechanisms. Careful diagnosis requires attention to the key symptom of androgen excess as well as the exclusion of similar disorders including hyperprolactinemia, late-onset congenital adrenal hyperplasia, and hypothalamic amenorrhea. A patient-centred approach to treatment requires further assessment of underlying drivers and mechanisms including neuroendocrine disturbance, insulin resistance, and adrenal hyperresponsiveness.
Key Words Overdiagnosis, neuroendocrine, hyperandrogenism, oral micronized progesterone
INTRODUCTION
The goal of health care is to alleviate symptoms and prevent negative health outcomes. To that end, medicine has become adept at categorizing and labelling constellations of symptoms in an attempt to deliver appropriate treatment. For some conditions, such an approach is highly effective, especially when there is a single and clearly identifiable underlying pathophysiology (e.g., infection). For other conditions, the approach is less effective, especially when there is multifactorial or heterogeneous pathophysiology (e.g., depression). If taken too far, enthusiastic diagnostic imaging and labelling of symptoms can result in overdiagnosis and overtreatment, as has been documented for depression, hyperlipidemia, and osteoporosis.1,2 Polycystic ovary syndrome (PCOS) is, unfortunately, both 1) a syndrome with a heterogeneous pathophysiology and 2) subject to overdiagnosis and overtreatment, largely as a result of the expansion3 of the Rotterdam diagnostic criteria to include non-androgenic PCOS.4
In this perspective article, I will outline how the current one-size-fits-all approach to diagnosing and treating PCOS can produce negative patient outcomes such as healthy women receiving an unnecessary disease label and the misdiagnosis of hypothalamic amenorrhea as PCOS. I will describe a more precise diagnostic approach which requires the key symptom of androgen excess and emphasizes the exclusion of disorders with similar clinical symptoms.
Furthermore, I will propose an individualized approach of “looking beyond the PCOS label” to identify the underlying physiological driver or drivers of hyperandrogenism in each patient. Such an approach will enable clinicians to mechanistically target the driver with evidence-based methods, such as myo-inositol, and deliver better patient-centred outcomes such as relieving androgen excess and a return to ovulatory cycles.
BACKGROUND
From the earliest descriptions in the medical literature, polycystic ovary syndrome (PCOS) was recognized as a condition of androgen excess, with high luteinizing hormone (LH) and testosterone levels generally regarded as key components of a diagnosis.3 Although an abnormal ovarian appearance was observed in some patients, it was not required for diagnosis, in part because pelvic ultrasound was not yet available.
The first formal PCOS diagnostic criteria from the National Institutes of Health (NIH)5 did not include ovarian morphology but, instead, characterized PCOS as unexplained hyperandrogenic anovulation. More precisely, the NIH criteria stated that PCOS can be diagnosed when all of the following criteria are present: symptoms of androgen excess (clinical or biochemical), infrequent ovulation, and exclusion of other disorders with similar clinical symptoms. This is consistent with the definition of “anovulatory androgen excess” later proposed by Canadian endocrinology professor Jerilynn C. Prior6 and the criteria of the Androgen Excess and PCOS (AE-PCOS) Society Task Force, which state that “PCOS should be defined by the presence of hyperandrogenism (clinical and/or biochemical), ovarian dysfunction (oligo-anovulation and/or polycystic ovaries), and the exclusion of related disorders.”7
Therefore, according to the NIH, Professor Jerilynn Prior, and the AE-PCOS Society, PCOS is, by definition, a condition of androgen excess. In simplest terms, PCOS is the symptom of androgen excess when all other causes of androgen excess have been ruled out.
That clear focus on androgen excess changed in 2004 with the introduction of the Rotterdam Criteria,4 which proposed for the first time polycystic ovarian morphology (PCOM) as a stand-alone criterion, thus enabling a PCOS diagnosis to be made based on only two of the following three criteria:
- rare ovulation or lack of ovulation,
- symptoms of androgen excess (clinical or biochemical),
- polycystic ovarian morphology.
This small but important change dramatically increased the incidence of PCOS from 5% of women of reproductive age to an incredible 20%8 and opened the door to the diagnosis of PCOS in the absence of androgen excess. According to the Rotterdam criteria, non-androgenic PCOS can be diagnosed based only on the symptoms of anovulation and polycystic ovaries, which equates to counting the same symptom twice because the polycystic (or more accurately, poly-follicular) appearance of the ovaries is merely an indicator of temporarily stalled follicle development and failure to ovulate in that cycle. In other words, anovulation (of any cause) is likely to correlate with PCOM, and the fact that anovulatory cycles are common (occurring in up to 30% of otherwise clinically normal menstrual cycles9) means that polycystic ovaries are common (occurring in up to 30% of women with normal cycles and hormones).10
The disconnect between the PCOM and androgen excess has been addressed in several papers including a 2010 commentary in The Journal of Clinical Endocrinology & Metabolism, which acknowledges that “an isolated PCOM in an ovulatory woman is not an indication for metabolic evaluation,”11 and a 2022 paper about diagnostic criteria,12 which notes that it is entirely possible to have the endocrine condition of androgenic PCOS but normal looking ovaries. The same paper goes on to say that “the presence of PCOM is neither necessary nor sufficient for diagnosis of PCOS” and concludes “there is no evidence that the presence of PCOM has any implications with regard to the endocrine or metabolic features of PCOS.”
It’s also worth noting that it is entirely possible to have androgenic PCOS but normal looking ovaries, especially in older women, who have fewer follicles.
So, polycystic ovaries occur in some (but not all) women with PCOS and in women with normal hormones. Polycystic ovaries also occur in women using hormonal birth control and women with any condition of oligo- or amenorrhea including the increasingly common scenario of hypothalamic amenorrhea or relative energy deficiency in sport (RED-s) as a result of undereating or underfueling. Of course, even under the Rotterdam Criteria, hypothalamic amenorrhea is supposed to be ruled out by asking patients about restricted eating. In practice, many clinicians do not ask about restricted eating, which means hypothalamic amenorrhea is frequently misdiagnosed as PCOS.13
The Problem of Misdiagnosis and Overdiagnosis
According to public health researcher Tessa Copp from The University of Sydney, the problem goes further than just misdiagnosis of hypothalamic amenorrhea as PCOS. In a recent British Medical Journal article titled “Driven by good intentions: Why widening the diagnostic criteria for polycystic ovary syndrome may be harming women,”14 Copp argues that even the tightened diagnostic criteria of the 2018 International PCOS Guidelines15 “still capture many women with few or mild symptoms,” including women who would naturally outgrow their androgen symptoms.16 Women who would naturally outgrow mild hyperandrogenism include young women and women experiencing what the author has clinically observed to be rebound hyperandrogenism upon discontinuation of androgen-suppressing medication such as cyproterone- and drospirenone-containing oral contraceptives.
Copp warns that in the case of mild or temporary PCOS, women are harmed by the unnecessary fear that “their condition will worsen, which threatens their perception of health and fertility.”14 As part of her research, Copp and her team conducted a qualitative study,17 which found that “fear of infertility can result in adverse psychological and behavioural consequences including anxiety, depression, lower self-worth, altered life and education goals, risk-taking with contraception, and unintended pregnancies.” In the same study, she called for “reducing the harms of unnecessarily labelling healthy women for whom the benefits of a diagnosis are small.” Yet another paper18 explored the subjective nature of assessing clinical hyperandrogenism (hirsutism) and warned that a “milder degree [of hirsutism] can be considered as normal patterns” for some ethnic groups and “not a reason for seeking medical care.”
An additional concern is when a PCOS diagnosis is provided to patients seeking an explanation for pelvic pain. According to a 2017 study,19 patients report pain as the most common symptom of PCOS. This is despite the fact that pain is not a symptom of PCOS.15 The reason for this mismatch between what the patient needs (an explanation for pain) and what is provided (a possibly mistaken and unnecessary PCOS diagnosis) is that both PCOS and menstrual pain are common, so it’s easy to have both a PCOS diagnosis and menstrual pain, including pain due to endometriosis.
Misdiagnosing hypothalamic amenorrhea and endometriosis as PCOS muddies the waters for both researchers and clinicians. In my own clinical practice, I commonly encounter patients who say: “I’ve tried everything for my PCOS but still have this pain.” By which they mean they have tried metformin and a low-carb diet but still have pain, which is not surprising given that neither metformin nor a low-carb diet is a treatment for pelvic pain.
Finally, it’s important to remember that PCOS is a “diagnosis of exclusion,”20 with all formal diagnostic criteria (NHI, AE-PCOS, and Rotterdam) stipulating the exclusion of other disorders with similar clinical symptoms.5,7,4 Clinicians should therefore take care to rule out other causes of anovulation and androgen excess including thyroid disease, Cushing syndrome, nonclassical or late-onset congenital adrenal hyperplasia (CAH), hyperprolactinemia, hypothalamic amenorrhea, and side effects from medication, such as progestins with a high androgen index.
Androgenic PCOS is a Heterogeneous Syndrome
Even when properly diagnosed, androgenic PCOS is still an “umbrella diagnosis,” which means a set of symptoms (i.e., a syndrome) resulting from one or more different underlying biological drivers or mechanisms. For context, another well-known umbrella diagnosis is irritable bowel syndrome (IBS), which is a set of digestive symptoms resulting from different underlying mechanisms including dysbiosis, food intolerances, altered intestinal motility, and more.21
The umbrella or heterogeneous nature of the PCOS diagnosis was acknowledged almost from the beginning, with a 2002 paper stating that “a single cause [of PCOS] is unlikely.”22 The Public Library of Science (PLOS) took it further in 2020, when they said that “PCOS is, in fact, a heterogeneous disorder with different underlying biological mechanisms” [emphasis mine] and that “grouping women with PCOS under a single diagnosis may be counterproductive because distinct disease subtypes will likely benefit from different interventions.”23
Treat the Individual
When treating a heterogeneous syndrome like PCOS, the best clinical strategy is to look beyond the diagnostic label of PCOS to possible underlying biological drivers and treat those. By doing so, androgen excess can be relieved and ovulatory cycles restored.
Documented physiological drivers of hyperandrogenism include neuroendocrine disturbance, insulin resistance, and adrenal hyperresponsiveness, all of which may be present simultaneously and interact.
Neuroendocrine Disturbance
In our paper, “The central role of ovulatory disturbances in the etiology of androgenic polycystic ovary syndrome,”24 my co-authors Professor Jerilynn Prior and Sonia Shirin and I build the case that the central disturbance in many cases of androgenic PCOS is abnormally rapid pulsatility of gonadotropin-releasing hormone (GnRH). Such a disturbance is downstream from various origins including chronic inflammation25 and genetic polymorphisms such as polymorphisms of the genes for FSH and LH receptors,26 and 17-α-hydroxylase/17–20 lyase,27 which is the rate-limiting step of androgen biosynthesis. Developmental factors are also important, especially in utero exposure to androgens,28 which causes epigenetic changes to the genes associated with GnRH pulsatility and steroidogenesis29 and creates a five-fold increased risk of androgenic PCOS in daughters of mothers with PCOS.30
Rapid GnRH pulsatility, in turn, promotes further impairment of the hypothalamic-pituitary-ovarian (HPO) axis by suppressing follicle-stimulating hormone (FSH) and stimulating LH resulting in stalled follicle development and high thecal cell androgen production, leading to aromatization and tonically high estrogen levels.24 The resulting ovulatory disturbance and progesterone deficiency further deprive the system of the progesterone feedback that normally slows GnRH pulsatility, creating a vicious cycle,24 as illustrated in Figure 1.
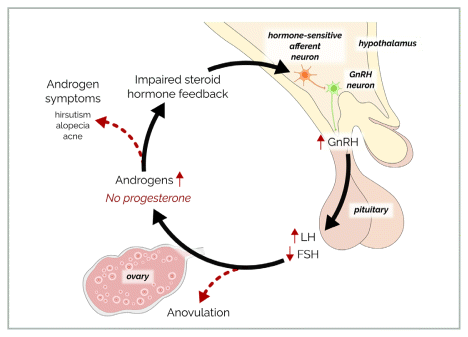
FIGURE 1 A model of the neuroendocrine disturbance causing androgen excess in some cases of androgenic PCOS. Dysregulation of the neural circuit of the GnRH/LH pulse generator leads to anovulation, absent progesterone, and consequent hyperandrogenism, creating a vicious cycle of impaired steroid feedback and further dysregulation of the neural circuit.
Assessing for Neuroendocrine Disturbance
The simplest way to clinically assess for rapid GnRH pulsatility is to measure serum LH and FSH on day two or three of the menstrual cycle, or, in the case of amenorrhea or no cycle, on a random day, taking care to not misinterpret a normal ovulatory surge of LH as elevated baseline LH. Androgenic PCOS with neuroendocrine disturbance typically presents with a high ratio (>2:1) of baseline serum LH to FSH.31 Hypothalamic amenorrhea typically presents with a normal to low ratio of baseline serum LH to FSH.31
Treatments or Interventions for Neuroendocrine Disturbance
The nutrient myo-inositol amplifies intracellular FSH signalling and promotes ovulation32; it can therefore help to correct the rapid GnRH pulsatility of androgenic PCOS. Myo-inositol is one of the evidence-based PCOS treatments included in the 2018 “Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome.”15
Cyclic progesterone therapy using oral micronized progesterone (OMP) given in a cyclic pattern for 14 days to mimic the luteal phase can also improve neuroendocrine disturbance by exerting beneficial negative feedback on GnRH pulsatility,24 as illustrated in Figure 2. Oral micronized progesterone induces withdrawal bleeds, slows the GnRH pulse generator, suppresses LH, lowers androgens, and eventually promotes ovulatory cycles.24 Mechanisms by which progesterone lowers androgens include 1) slowing of the GnRH pulse generator, thereby reducing the LH stimulation of thecal cells and 2) competing for the enzyme 5 alpha-reductase, thereby reducing dihydrotestosterone (DHT), which is the active form of testosterone. Cyclic progesterone therapy is currently undergoing a clinical trial for PCOS.33
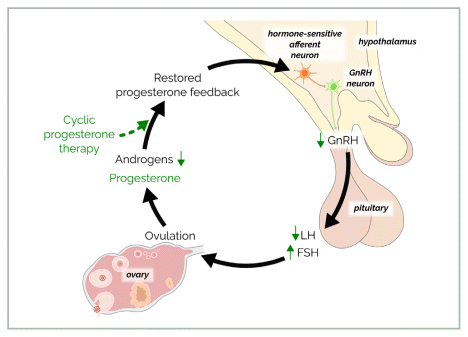
FIGURE 2 Treatment of androgenic polycystic ovary syndrome with cyclic oral micronized progesterone therapy. Oral micronized progesterone (OMP) given in a cyclic pattern for 14 days to mimic the luteal phase acts centrally to restore the healthy function of the GnRH pulse generator, suppress LH, and reduce androgen excess, promoting ovulation.
Insulin Resistance
A second driver of androgenic PCOS is insulin resistance or hyperinsulinemia, which contributes to androgen excess by:
- Directly stimulating ovarian thecal cells to produce androgens,34 possibly by acting as a co-gonadotropin with LH35
- Increasing unbound or free testosterone by decreasing sex hormone-binding globulin (SHBG)
- Contributing to neuroendocrine disturbance by increasing GnRH pulsatility.36
Thus, hyperinsulinemia contributes to the vicious cycle of the first biological driver (neuroendocrine disturbance) to a greater or lesser degree depending on the severity. Severe hyperinsulinemia can even produce androgen excess in the absence of neuroendocrine disturbance or other source of hyperandrogenism.37
Hyperinsulinemia is, however, unlikely to be the primary initiating driver for most women with androgenic PCOS. Instead, insulin resistance is likely to be secondary to neuroendocrine anovulatory hyperandrogenism based on the findings that the androgenic anovulatory PCOS phenotypes have worse insulin sensitivity than do ovulatory phenotypes38,39,40,41 and that increasing androgen burden often leads to a deterioration of insulin sensitivity.42 Anovulatory hyperandrogenism was further demonstrated to be causal by a prospective study of a random sample of adolescents, which found that PCOS by age 18 was best predicted by oligomenorrhea at age 14, and not by obesity or insulin resistance.43 In other words, androgen excess probably comes first and promotes insulin resistance,44,45 via several mechanisms, including deposition of visceral fat,46,47 the “masculinization” of adipose tissue,48 decreased muscle glucose uptake,49 decreased thermogenesis and energy expenditure,50 and lipid accumulation in liver.51
Assessing for Insulin Resistance
Signs and symptoms of insulin resistance include a high waist measure, acanthosis nigricans, low HDL cholesterol, and high serum triglycerides. Other possible laboratory findings include high HbA1C, high fasting insulin,52 and, most accurately, a high HOMA-IR index (homeostatic model for insulin resistance), which is a calculation of fasting glucose and insulin concentrations, defined as fasting plasma glucose (mmol/L) × fasting plasma insulin (μU/mL/22.5).53 A recent study of HOMA-IR and insulin resistance concluded that “monitoring insulin may have clinical relevance,” including in patients with normal fasting glucose.54 And in a study of girls with PCOS, fasting insulin was identified as a simple blood test that can be used clinically to guide treatment.55 Of note, a positive response to insulin-sensitizing medication has been observed in normal-weight women with PCOS,56 suggesting that mild insulin resistance is a factor even in so-called “lean PCOS.”
Treatment or Intervention for Insulin Resistance
Conventional treatment for insulin resistance includes diet, exercise, and insulin-sensitizing medication, such as metformin. Nutritional supplements that have been clinically trialled for insulin resistance include magnesium,57 myo-inositol,58 and the phytonutrients berberine59 and silymarin.60
Adrenal Hyperresponsiveness
Most women with PCOS have an elevation of all types of androgens: testosterone from the ovaries, androstenedione from the ovaries and adrenal glands, and DHEA-S (dehydroepiandrosterone sulfate) from the adrenal glands. When only DHEA-S is elevated but testosterone and androstenedione are normal, it may be classified as the adrenal subtype of PCOS,61 which researchers have described as “a subclinical form of micronodular bilateral adrenal hyperplasia,”62 and which accounts for about 10% of PCOS diagnoses.7 Adrenal PCOS is driven by hyperresponsiveness of the adrenal cortex and stress response system,63 and may be the result of stress or trauma around the time of puberty.64
Assessing for Adrenal PCOS
The adrenal PCOS sub-type is suggested by elevated serum DHEA-S, together with normal testosterone and androstenedione. A similar condition of nonclassical or late-onset CAH should be ruled out by screening for elevated follicular phase 17-OH progesterone, followed by an adrenocorticotropic hormone (ACTH) stimulation test and genetic testing. Late-onset CAH accounts for up to 9% of cases of androgen excess and can be misdiagnosed as PCOS.65
Treatment or Intervention for Adrenal Hyperresponsiveness
Adrenal PCOS may benefit from nutrients and herbal medicines that support the stress response system, particularly pantothenic acid, which helps to modulate the response of the adrenal cortex to ACTH.66 Androgen symptoms can also be improved with progesterone, either by taking cyclic progesterone therapy or by promoting ovulatory cycles and robust luteal phases. Finally, adrenal androgens can be downregulated with hydrocortisone67 and the herbal medicine licorice (Glycyrrhiza glabra), which slows the activity of 17-α-hydroxylase, thereby lowering androgens68 and “could be considered as an adjuvant therapy for hirsutism and polycystic ovary syndrome.”68
CONCLUSION
The first step in a patient-centred approach to PCOS is careful diagnosis, with attention to the key symptom of hyperandrogenism and exclusion of disorders with similar clinical symptoms such as hyperprolactinemia, thyroid disease, and hypothalamic amenorrhea. By focussing on hyperandrogenism, and avoiding non-androgenic PCOS phenotypes, clinicians can avoid the harms of labelling healthy women or women with hypothalamic amenorrhea with an unnecessary (and possibly inaccurate) PCOS diagnosis.
The next step in a patient-centred approach is to recognize that PCOS is not a single disease but is instead a heterogeneous syndrome with multiple underlying physiological drivers, including neuroendocrine disturbance, insulin resistance, and adrenal hyperresponsiveness. By looking beyond the disease label to the underlying physiological driver, clinicians can mechanistically target the driver with evidence-based methods such as myo-inositol and cyclic progesterone therapy. Such an approach can produce the desirable patient outcomes of relieving androgen excess and a return to ovulatory cycles.
AUTHOR AFFILIATIONS
1Centre for Menstrual Cycle and Ovulation Research (CeMCOR), University of British Columbia, Vancouver, BC, Canada.
ACKNOWLEDGEMENTS
Not applicable.
CONFLICTS OF INTEREST DISCLOSURE
I have read and understood the CAND Journal’s policy on conflicts of interest and declare that I have none.
FUNDING
This research did not receive any funding.
REFERENCES
1. Kale MS, Korenstein D. Overdiagnosis in primary care: framing the problem and finding solutions. BMJ. 2018;362:k2820. https://doi.org/10.1136/bmj.k2820
Crossref
2. Mintzes B, Swandari S, Fabbri A, Grundy Q, Moynihan R, Bero L. Does industry-sponsored education foster overdiagnosis and overtreatment of depression, osteoporosis and over-active bladder syndrome? An Australian cohort study. BMJ Open. 2018;8(2):e019027. https://doi.org/10.1136/bmjopen-2017-019027
Crossref
3. History of discovery of polycystic ovary syndrome. Adv Clin Exp Med. 2017;26(3):555–558. https://doi.org/10.17219/acem/61987
Crossref
4. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. https://doi.org/10.1016/j.fertnstert.2003.10.004
Crossref
5. Zawadzki J, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens HR, Haseltine FP, Merriam GR, ed. Polycystic ovary syndrome. Blackwell Scientific; 1992:377–384.
6. Prior JC, Kalyan S, Seifert-Klauss V. Re-naming PCOS: suggest anovulatory androgen excess (Letter to the editor). J Clin Endocrinol Metabol. 2013;98:4325–4328.
7. Azziz R, Carmina E, Dewailly D, et al. The androgen excess and PCOS society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91(2):456–488. https://doi.org/10.1016/j.fertnstert.2008.06.035
Crossref
8. Wilcock J, Taylor D. Polycystic ovarian syndrome: overdiagnosed and overtreated?. Br J Gen Pract. 2018;68(670):243. https://doi.org/10.3399/bjgp18X696113
Crossref
9. Prior JC, Naess M, Langhammer A, Forsmo S. Ovulation prevalence in women with spontaneous normal-length menstrual cycles—A population-based cohort from HUNT3, Norway. PLOS One. 2015;10(8):e0134473. https://doi.org/10.1371/journal.pone.0134473
Crossref
10. Polson DW, Adams J, Wadsworth J, Franks S. Polycystic ovaries—a common finding in normal women. Lancet. 1988;1(8590):870–872. https://doi.org/10.1016/s0140-6736(88)91612-1
Crossref
11. Johnstone EB, Rosen MP, Neril R, et al. The polycystic ovary post-Rotterdam: a common, age-dependent finding in ovulatory women without metabolic significance. J Clin Endocrinol Metab. 2010;95(11):4965–4972. https://doi.org/10.1210/jc.2010-0202
Crossref
12. Chang S, Dunaif A. Diagnosis of polycystic ovary syndrome: which criteria to use and when?. Endocrinol Metab Clin North Am. 2021;50(1):11–23. https://doi.org/10.1016/j.ecl.2020.10.002
Crossref
13. Alemyar A, van der Kooi ALF, Laven JSE. Anti-Müllerian hormone and ovarian morphology in women with hypothalamic hypogonadism. J Clin Endocrinol Metab. 2020;105(5). https://doi.org/10.1210/clinem/dgaa116
Crossref
14. Copp T, Doust J, McCaffery K, Hersch J, Jansen J. Polycystic ovary syndrome: why widening the diagnostic criteria may be harming women. BMJ. 2021;373:n700. https://doi.org/10.1136/bmj.n700
Crossref
15. Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110(3):364–379. https://doi.org/10.1016/j.fertnstert.2018.05.004
Crossref
16. Copp T, Jansen J, Doust J, Mol BW, Dokras A, McCaffery K. Are expanding disease definitions unnecessarily labelling women with polycystic ovary syndrome?. BMJ. 2017;358:j3694. https://doi.org/10.1136/bmj.j3694
Crossref
17. Copp T, Hersch J, Muscat DM, et al. The benefits and harms of receiving a polycystic ovary syndrome diagnosis: a qualitative study of women’s experiences. Hum Reprod Open. 2019;2019(4):hoz026. https://doi.org/10.1093/hropen/hoz026
Crossref
18. Soares-Jr JM, Sá MFS, Baracat EC. New criteria for the clinical diagnosis of hyperandrogenism in polycystic ovarian syndrome and the risk of overdiagnosis. Rev Bras Ginecol Obstet. 2019;41(6):361–362. https://doi.org/10.1055/s-0039-1693530
Crossref
19. Martin ML, Halling K, Eek D, Krohe M, Paty J. Understanding polycystic ovary syndrome from the patient perspective: a concept elicitation patient interview study. Health Qual Life Outcomes. 2017;15(1):162. https://doi.org/10.1186/s12955-017-0736-3
Crossref
20. Kyritsi EM, Dimitriadis GK, Kyrou I, Kaltsas G, Randeva HS. PCOS remains a diagnosis of exclusion: a concise review of key endocrinopathies to exclude. Clin Endocrinol (Oxf). 2017;86(1):1–6. https://doi.org/10.1111/cen.13245
Crossref
21. Bellini M, Gambaccini D, Stasi C, Urbano MT, Marchi S, Usai-Satta P. Irritable bowel syndrome: a disease still searching for pathogenesis, diagnosis and therapy. World J Gastroenterol. 2014;20(27):8807–8820. https://doi.org/10.3748/wjg.v20.i27.8807
22. Balen A, Michelmore K. What is polycystic ovary syndrome? Are national views important?. Hum Reprod. 2002;17(9):2219–2227. https://doi.org/10.1093/humrep/17.9.2219
Crossref
23. Dapas M, Lin FTJ, Nadkarni GN, et al. Distinct subtypes of polycystic ovary syndrome with novel genetic associations: an unsupervised, phenotypic clustering analysis. PLOS Med. 2020;17(6):e1003132. https://doi.org/10.1371/journal.pmed.1003132
Crossref
24. Briden L, Shirin S, Prior JC. The central role of ovulatory disturbances in the etiology of androgenic polycystic ovary syndrome (PCOS)—evidence for treatment with cyclic progesterone. Drug Discov Today Dis Model. 2020;32:71–82.
Crossref
25. Bressler BH, Dusik LA, Menard MR. Tension responses of frog skeletal muscle fibres to rapid shortening and lengthening steps. J Physiol. 1988;397:631–641. https://doi.org/10.1113/jphysiol.1988.sp017022
Crossref
26. Hayes MG, Urbanek M, Ehrmann DA, et al. Publisher correction: genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations. Nat Commun. 2020;11(1):2158. https://doi.org/10.1038/s41467-020-15793-w
Crossref
27. Xu X, Hu K, Shi H, Yu Y, Xu J, Sun Y. The single-nucleotide polymorphism rs743572 of CYP17A1 shows significant association with polycystic ovary syndrome: a meta-analysis. Reprod Biomed Online. 2021;43(5):941–951. https://doi.org/10.1016/j.rbmo.2021.06.012
Crossref
28. Falbo A, Rocca M, Russo T, et al. Changes in androgens and insulin sensitivity indexes throughout pregnancy in women with polycystic ovary syndrome (PCOS): relationships with adverse outcomes. J Ovarian Res. 2010;3:23. https://doi.org/10.1186/1757-2215-3-23
Crossref
29. Dumesic DA, Abbott DH, Padmanabhan V. Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord. 2007;8(2):127–141. https://doi.org/10.1007/s11154-007-9046-0
Crossref
30. Risal S, Pei Y, Lu H, et al. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat Med. 2019;25(12):1894–1904. https://doi.org/10.1038/s41591-019-0666-1
Crossref
31. Abou Sherif S, Newman R, Haboosh S, et al. Investigating the potential of clinical and biochemical markers to differentiate between functional hypothalamic amenorrhoea and polycystic ovarian syndrome: a retrospective observational study. Clin Endocrinol (Oxf). 2021;95(4):618–627. https://doi.org/10.1111/cen.14571
Crossref
32. Facchinetti F, Unfer V, Dewailly D, et al. Inositols in polycystic ovary syndrome: an overview on the advances. Trends Endocrinol Metab. 2020;31(6):435–447. https://doi.org/10.1016/j.tem.2020.02.002
Crossref
33. Prior JC, Singer J, Goshtasebi A, et al. Phase II 6-month cyclic progesterone/spironolactone pilot therapy trial in polycystic ovary syndrome: pre-post, single-arm feasibility study. 2022. The University of British Columbia. https://doi.org/10.14288/1.0396377. Retrieved from https://open.library.ubc.ca/soa/cIRcle/collections/facultyresearchandpublications/52383/items/1.0396377
34. Qu J, Wang Y, Wu X, Gao L, Hou L, Erkkola R. Insulin resistance directly contributes to androgenic potential within ovarian theca cells. Fertil Steril. 2009;91(5 Suppl):1990–1997. https://doi.org/10.1016/j.fertnstert.2008.02.167
Crossref
35. Barbieri RL, Smith S, Ryan KJ. The role of hyperinsulinemia in the pathogenesis of ovarian hyperandrogenism. Fertil Steril. 1988;50(2):197–212. https://doi.org/10.1016/s0015-0282(16)60060-2
Crossref
36. Kim HH, DiVall SA, Deneau RM, Wolfe A. Insulin regulation of GnRH gene expression through MAP kinase signaling pathways. Mol Cell Endocrinol. 2005;242(1–2):42–49. https://doi.org/10.1016/j.mce.2005.07.002
Crossref
37. Rager KM, Omar HA. Androgen excess disorders in women: the severe insulin-resistant hyperandrogenic syndrome, HAIR-AN. Sci World J. 2006;6:116–121. https://doi.org/10.1100/tsw.2006.23
Crossref
38. Panidis D, Tziomalos K, Misichronis G, et al. Insulin resistance and endocrine characteristics of the different phenotypes of polycystic ovary syndrome: a prospective study. Hum Reprod. 2012;27(2):541–549. https://doi.org/10.1093/humrep/der418
Crossref
39. Diamanti-Kandarakis E, Panidis D. Unravelling the phenotypic map of polycystic ovary syndrome (PCOS): a prospective study of 634 women with PCOS. Clin Endocrinol (Oxf). 2007;67(5):735–742. https://doi.org/10.1111/j.1365-2265.2007.02954.x
Crossref
40. Kauffman RP, Baker TE, Baker VM, DiMarino P, Castracane VD. Endocrine and metabolic differences among phenotypic expressions of polycystic ovary syndrome according to the 2003 Rotterdam consensus criteria. Am J Obstet Gynecol. 2008;198(6):670.e1–e10. https://doi.org/10.1016/j.ajog.2008.01.037
Crossref
41. Wiltgen D, Spritzer PM. Variation in metabolic and cardiovascular risk in women with different polycystic ovary syndrome phenotypes. Fertil Steril. 2010;94(6):2493–2496. https://doi.org/10.1016/j.fertnstert.2010.02.015
Crossref
42. O’Reilly MW, Taylor AE, Crabtree NJ, et al. Hyperandrogenemia predicts metabolic phenotype in polycystic ovary syndrome: the utility of serum androstenedione. J Clin Endocrin Metabol. 2014;99(3):1027–1036. https://doi.org/10.1210/jc.2013-3399
Crossref
43. van Hooff MH, Voorhorst FJ, Kaptein MB, Hirasing RA, Koppenaal C, Schoemaker J. Predictive value of menstrual cycle pattern, body mass index, hormone levels and polycystic ovaries at age 15 years for oligo-amenorrhoea at age 18 years. Hum Reprod. 2004;19(2):383–392. https://doi.org/10.1093/humrep/deh079
Crossref
44. Diamanti-Kandarakis E, Papalou O, Kandaraki EA. The role of androgen excess on insulin sensitivity in women. Front Horm Res. 2019;53:50–64. https://doi.org/10.1159/000494902
Crossref
45. Ruth KS, Day FR, Tyrrell J, et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med. 2020;26(2):252–258. https://doi.org/10.1038/s41591-020-0751-5
Crossref
46. Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36(5):487–525. https://doi.org/10.1210/er.2015-1018
Crossref
47. Borruel S, Fernández-Durán E, Alpañés M, et al. Global adiposity and thickness of intraperitoneal and mesenteric adipose tissue depots are increased in women with polycystic ovary syndrome (PCOS). J Clin Endocrinol Metab. 2013;98(3):1254–1263. https://doi.org/10.1210/jc.2012-3698
Crossref
48. Montes-Nieto R, Insenser M, Martínez-García MÁ, Escobar-Morreale HF. A nontargeted proteomic study of the influence of androgen excess on human visceral and subcutaneous adipose tissue proteomes. J Clin Endocrinol Metab. 2013;98(3):E576–E585. https://doi.org/10.1210/jc.2012-3438
Crossref
49. Inada A, Fujii NL, Inada O, Higaki Y, Furuichi Y, Nabeshima YI. Effects of 17β-estradiol and androgen on glucose metabolism in skeletal muscle. Endocrinology. 2016;157(12):4691–4705. https://doi.org/10.1210/en.2016-1261
Crossref
50. Morford J, Mauvais-Jarvis F. Sex differences in the effects of androgens acting in the central nervous system on metabolism. Dialogues Clin Neurosci. 2016;18(4):415–424. https://pubmed.ncbi.nlm.nih.gov/28179813
Crossref
51. Kanaya N, Vonderfecht S, Chen S. Androgen (dihydrotestosterone)-mediated regulation of food intake and obesity in female mice. J Steroid Biochem Mol Biol. 2013;138:100–106. https://doi.org/10.1016/j.jsbmb.2013.04.001
Crossref
52. Zhang X, Li J, Zheng S, Luo Q, Zhou C, Wang C. Fasting insulin, insulin resistance, and risk of cardiovascular or all-cause mortality in non-diabetic adults: a meta-analysis. Biosci Rep. 2017;37(5). https://doi.org/10.1042/BSR20170947
Crossref
53. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. https://doi.org/10.2337/diacare.27.6.1487
Crossref
54. Caporaso NE, Jones RR, Stolzenberg-Solomon RZ, Medgyesi DN, Kahle LL, Graubard BI. Insulin resistance in healthy U.S. adults: findings from the National Health and Nutrition Examination Survey (NHANES). Cancer Epidemiol Biomarkers Prev. 2020;29(1):157–168. https://doi.org/10.1158/1055-9965.EPI-19-0206
Crossref
55. Cree-Green M, Cai N, Thurston JE, et al. Using simple clinical measures to predict insulin resistance or hyperglycemia in girls with polycystic ovarian syndrome. Pediatr Diabetes. 2018;19(8):1370–1378. https://doi.org/10.1111/pedi.12778
Crossref
56. Nestler JE, Jakubowicz DJ. Lean women with polycystic ovary syndrome respond to insulin reduction with decreases in ovarian P450c17 alpha activity and serum androgens. J Clin Endocrinol Metab. 1997;82(12):4075–4079. https://doi.org/10.1210/jcem.82.12.4431
57. Morais JBS, Severo JS, de Alencar GRR, et al. Effect of magnesium supplementation on insulin resistance in humans: a systematic review. Nutrition. 2017;38:54–60. https://doi.org/10.1016/j.nut.2017.01.009
Crossref
58. Fruzzetti F, Perini D, Russo M, Bucci F, Gadducci A. Comparison of two insulin sensitizers, metformin and myo-inositol, in women with polycystic ovary syndrome (PCOS). Gynecol Endocrinol. 2017;33(1):39–42. https://doi.org/10.1080/09513590.2016.1236078
Crossref
59. Zhao H, Xing C, Zhang J, He B. Comparative efficacy of oral insulin sensitizers metformin, thiazolidinediones, inositol, and berberine in improving endocrine and metabolic profiles in women with PCOS: a network meta-analysis. Reprod Health. 2021;18(1):171. https://doi.org/10.1186/s12978-021-01207-7
Crossref
60. MacDonald-Ramos K, Michán L, Martínez-Ibarra A, Cerbón M. Silymarin is an ally against insulin resistance: a review. Ann Hepatol. 2021;23:100255. https://doi.org/10.1016/j.aohep.2020.08.072
Crossref
61. Gourgari E, Lodish M, Keil M, et al. Bilateral adrenal hyperplasia as a possible mechanism for hyperandrogenism in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2016;101(9):3353–3360. https://doi.org/10.1210/jc.2015-4019
Crossref
62. Hoffman DI, Klove K, Lobo RA. The prevalence and significance of elevated dehydroepiandrosterone sulfate levels in anovulatory women. Fertil Steril. 1984;42(1):76–81. https://doi.org/10.1016/s0015-0282(16)47961-6
Crossref
63. Rasmusson AM, Vasek J, Lipschitz DS, et al. An increased capacity for adrenal DHEA release is associated with decreased avoidance and negative mood symptoms in women with PTSD. Neuropsychopharmacology. 2004;29(8):1546–1557. https://doi.org/10.1038/sj.npp.1300432
Crossref
64. Lobo RA, Granger LR, Paul WL, Goebelsmann U, Mishell DR Jr. Psychological stress and increases in urinary norepinephrine metabolites, platelet serotonin, and adrenal androgens in women with polycystic ovary syndrome. Am J Obstet Gynecol. 1983;145(4):496–503. https://doi.org/10.1016/0002-9378(83)90324-1
Crossref
65. Witchel SF. Nonclassic congenital adrenal hyperplasia. Curr Opin Endocrinol Diabetes Obes. 2012;19(3):151–158. https://doi.org/10.1097/MED.0b013e3283534db2
Crossref
66. Pan L, Jaroenporn S, Yamamoto T, et al. Effects of pantothenic acid supplement on secretion of steroids by the adrenal cortex in female rats. Reprod Med Biol. 2012;11(2):101–104. https://doi.org/10.1007/s12522-011-0113-6
Crossref
67. Rezvani I, Garibaldi LR, Digeorge AM, Artman HG. Disproportionate suppression of dehydroepiandrosterone sulfate (DHEAS) in treated patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatr Res. 1983;17(2):131–134. https://doi.org/10.1203/00006450-198302000-00010
Crossref
68. Armanini D, Mattarello MJ, Fiore C, et al. Licorice reduces serum testosterone in healthy women. Steroids. 2004;69(11–12):763–766. https://doi.org/10.1016/j.steroids.2004.09.005
Crossref
Correspondence to: Lara Briden, Centre for Menstrual Cycle and Ovulation Research, in Endocrinology, University of British Columbia, 2775 Laurel Street, Vancouver, BC V5Z 1M9, Canada. E-mail: lara@larabriden.com
To cite: Briden L. Beyond the label: A patient-centred approach to polycystic ovary syndrome. CAND Journal. 2022;29(2):2-8. https:/doi.org/10.54434/candj.114
Received: 09 March 2022; Accepted: 06 May 2022; Published: 28 June 2022
© 2022 Canadian Association of Naturopathic Doctors. For permissions, please contact candj@cand.ca.
CAND Journal | Volume 29, No. 2, June 2022
(Return to Top)